Objectives
To help students to
i) recall the formula of a given chemical compound
ii) identify the metallic and non-metallic radicals
iii) frame the formula by matching the cards appropriately
Materials required
A chart displaying the names of the following chemical compounds (50), Aluminium oxide,
Silver nitrate.
Silver chloride
Aluminium hydroxide
Carbon monoxide
Calcium oxide
Copper oxide
Carbon dioxide
Copper carbonate
Calcium carbonate
Magnesium oxide
Manganese dioxide
Magnesium carbonate
Magnesium chloride
Magnesium hydroxide
Magnesium bicarbonate
Sodium oxide
Nitrogen dioxide |
Sodium carbonate
Sodium nitrate
Copper sulphate
Methane
Calcium chloride
Calcium hydroxide
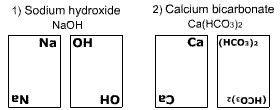
Calcium bicarbonate
Ferric chloride
Ferric hydroxide
Ferrous sulphate
Mercuric oxide
Zinc oxide
Sulphuric acid
Nitric acid
Hydrochloric acid
Carbonic acid |
Phosphoric acid
Sulphurous acid
Potassium oxide
Potassium hydroxide
Potassium chlorate
Potassium permanganate
Water
Sodium chloride
Ammonia
Ammonium chloride
Sodium hydroxide
Sodium bicarbonate
Ammonium hydroxide
Phosphorous pentoxide
Sulphur dioxide
Zinc chloride
|
- 100 discarded visiting cards
On each pair of cards, the symbolic representation of the metallic and nonmetallic radical of the coumpound is written.
Example:
Instruction to teachers
- Shuffle all the cards and distribute 10 to each of the students.
- Stack the rest of them face down
- Display the chart containing the names of the chemical compounds
- Draw the attention of the students towards the chart and tell that the formula of any of these compounds can be arranged using the matching cards.
- Ask the students to match one metallic radical with one non-metallic radical (from the given set of 10 cards) to form a compound. If the match is made correctly, the pair is kept aside. If no set can be made from it, the player picks up one card from the pile to make a match . Here again if it does not help, the card is put back, face up.
- Once a student makes a pair, check whether the combination is correct.
- Ask the students to take turns to pick up cards to make the sets.
The student who make the maximum number of pairs is the winner.
|